Radium chloride
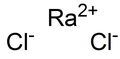
| |
 An ampoule containing radium chloride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.020 |
| EC Number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| RaCl2 | |
| Molar mass | 296.094 g/mol |
| Appearance | Colorless solid, glows blue-green[1] |
| Density | 4.9 g/cm3[1] |
| Melting point | 900 °C (1,650 °F; 1,170 K)[1] |
| 245 g/L (20 °C)[2] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
radioactive, highly toxic, corrosive |
| GHS labelling: | |
  
| |
| H300, H310, H330, H350, H370, H373, H410 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Radium bromide |
Other cations
|
Beryllium chloride Magnesium chloride Calcium chloride Strontium chloride Barium chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Radium chloride is an inorganic compound with the chemical formula RaCl2. It is a radium salt of hydrogen chloride. It was the first radium compound isolated in a pure state. Marie Curie and André-Louis Debierne used it in their original separation of radium from barium.[3] The first preparation of radium metal was by the electrolysis of a solution of this salt using a mercury cathode.[4]
Preparation
[edit]Radium chloride crystallises from aqueous solution as the dihydrate. The dihydrate is dehydrated by heating to 100 °C in air for one hour followed by 5.5 hours at 520 °C under argon.[5] If the presence of other anions is suspected, the dehydration may be effectuated by fusion under hydrogen chloride.[6]
Radium chloride can also be prepared by heating radium bromide in a flow of dry hydrogen chloride gas. It can be produced by treating radium carbonate with hydrochloric acid.
Properties
[edit]Radium chloride is a colorless salt with a blue-green luminescence, especially when heated. Its color gradually changes to yellow with aging, whereas contamination by barium may impart a rose tint.[1] It is less soluble in water than other alkaline earth metal chlorides – at 25 °C its solubility is 245 g/L whereas that of barium chloride is 307 g/L, and the difference is even larger in hydrochloric acid solutions. This property is used in the first stages of the separation of radium from barium by fractional crystallization.[2] Radium chloride is only sparingly soluble in azeotropic hydrochloric acid and virtually insoluble in concentrated hydrochloric acid.[7]
Gaseous RaCl2 shows strong absorptions in the visible spectrum at 676.3 nm and 649.8 nm (red): the dissociation energy of the radium–chlorine bond is estimated as 2.9 eV,[8] and its length as 292 pm.[9]
Contrary to diamagnetic barium chloride, radium chloride is weakly paramagnetic with a magnetic susceptibility of 1.05×106. Its flame color is red.[1]
Uses
[edit]Radium chloride is still used for the initial stages of the separation of radium from barium during the extraction of radium from pitchblende. The large quantities of material involved (to extract a gram of pure radium metal, about 7 tonnes of pitchblende is required) favour this less costly (but less efficient) method over those based on radium bromide or radium chromate (used for the later stages of the separation).
It was also used in medicine to produce radon gas which in turn was used as a brachytheraputic cancer treatment.[10][11]
Radium-223 dichloride (USP, radium chloride Ra 223), tradename Xofigo (formerly Alpharadin), is an alpha-emitting radiopharmaceutical. Bayer received FDA approval for this drug to treat prostate cancer osteoblastic bone metastases in May 2013. Radium-223 chloride is one of the most potent ((antineoplastic drugs)) known.[citation needed] One dose (50 kBq/kg) in an adult is about 60 nanograms; this amount is 1/1000 the weight of an eyelash (75 micrograms).
References
[edit]- ^ a b c d e Kirby, p. 5
- ^ a b Kirby, p. 6
- ^ Curie, M.; Debierne, A. (1910). C. R. Hebd. Acad. Sci. Paris 151:523–25.
- ^ Kirby, p. 3
- ^ Weigel, F.; Trinkl, A. (1968). "Crystal Chemistry of Radium. I. Radium Halides". Radiochimica Acta. 9: 36–41. doi:10.1524/ract.1968.9.1.36. S2CID 201843329.
- ^ Hönigschmid, O.; Sachtleben, R. (1934). "Revision des Atomgewichtes des Radiums". Zeitschrift für anorganische und allgemeine Chemie. 221: 65–82. doi:10.1002/zaac.19342210113.
- ^ Erbacher, Otto (1930). "Löslichkeits-Bestimmungen einiger Radiumsalze". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 63: 141–156. doi:10.1002/cber.19300630120.
- ^ Lagerqvist, A. (1953). Arkiv Fisik 6:141–42.
- ^ Karapet'yants, M. Kh.; Ch'ing, Ling-T'ing (1960). Zh. Strukt. Khim. 1:277–85; J. Struct. Chem. (USSR) 1:255–63.
- ^ Goldstein, N. (1975). "Radon seed implants. Residual radioactivity after 33 years". Archives of Dermatology. 111 (6): 757–759. doi:10.1001/archderm.1975.01630180085013. PMID 1137421.
- ^ Winston, P. (June 1958). "Carcinoma of the Trachea Treated by Radon Seed Implantation". The Journal of Laryngology & Otology. 72 (6): 496–499. doi:10.1017/S0022215100054232. PMID 13564019. S2CID 36790323.
Bibliography
[edit]- Kirby, H. W. and Salutsky, Murrell L. (1964) The Radiochemistry of Radium, Subcommittee on Radiochemistry, National Academy of Sciences
Sources
[edit]- Gmelins Handbuch der anorganischen Chemie (8. Aufl.), Berlin:Verlag Chemie, 1928, pp. 60–61.
- Gmelin Handbuch der anorganischen Chemie (8. Aufl. 2. Erg.-Bd.), Berlin:Springer, 1977, pp. 362–64.

